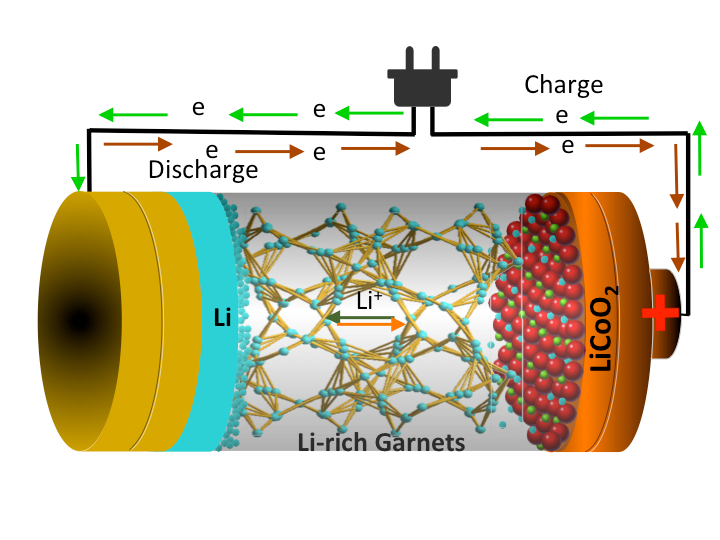
This is an illustration of a Li-rich garnet structure based all-solid-state-Li battery.
Jan. 19, 2017
Electric vehicles promise to revolutionize transportation but they need safer, better-performing batteries.
Now, a Faculty of Science researcher at the University of Calgary, and colleagues at the University of Maryland (UMD), College Park in the United States have made major inroads toward developing a next-generation, all solid-state lithium battery. Batteries are electrochemical devices that store energy in the form of chemical energy and deliver electricity when needed.
Venkataraman Thangadurai, professor in the Chemistry Department, worked with project leader Eric Wachsman, professor of engineering at UMD, and other scientists at UMD to build the advanced battery.
Their paper, “Negating Interfacial Impedance in Garnet-Based Solid-State Li Metal Batteries,” is published in Nature Materials, one of the top-ranked Nature series of journals.
Current lithium-ion batteries have several issues, including leakage, poor chemical stability, flammability, and limited operating voltage or energy density.
But the UCalgary-UMD research team’s new button-sized lithium battery is chemically stable, non-flammable, and can operate safely at a higher voltage than existing batteries.
“The technology we have developed would enable absolutely stable, robust, safe, high-powered, all solid-state lithium batteries for future energy storage,” Thangadurai says.
Such next-generation batteries have many potential applications, including in electric vehicles, consumer electronics, solid-state gas sensors, and electrical grids for storing power generated by renewable energy and providing electricity during peak demand times.
Engineering a better battery
Existing lithium-ion batteries like those used in the Tesla Motors cars, Chevrolet Volt, Nissan Leaf and other electric and plug-in hybrid vehicles, as well as in portable electronics, use membranes of organic polymer compounds and lithium salts as the electrolyte.
The electrolyte in a battery separates the two electrodes (the positive cathode and the negative anode), and conducts the lithium ions between the electrodes during charging and discharging cycles. Currently used organic polymer-based electrolytes are flammable, so fire is a safety issue.
Instead of organic polymers for their battery, Wachsman and co-principal investigators Thangadurai and Liangbing Hu (at the UMD), along with other UMD scientists, used a solid ceramic electrolyte, which doesn’t burn.
The research team also used, for the first time, a technique called atomic layer deposition to place a thin film of aluminum oxide on top of a garnet structure coating the ceramic electrolyte.

This is an illustration of a Li-rich garnet structure based all-solid-state-Li battery.
With their chemically engineered, garnet-based, ceramic electrolyte, the team showed there was “negligible interface resistance” between the lithium metal anode and the ceramic electrolyte interface. This resulted in fast transport of charges and higher performance overall.
Their newly developed interface chemistry also enabled the team to use a lithium metal anode and a high-voltage cathode, which will significantly boost their battery’s energy storage capacity and operating voltage compared with existing lithium-ion batteries.
While other research groups in the world have used garnet to build lithium batteries, “We showed we can use the lithium metal very efficiently, with the lowest interface-charge transfer resistance between the lithium electrode and the garnet electrolyte,” Thangadurai says.
UCalgary researcher pioneered the use of garnet
Thangadurai pioneered the use of garnet-type ceramics (a silicate mineral structure) in solid-state lithium batteries several years ago, while working as a postdoctoral researcher at the University of Kiel in Germany.
About three years ago, he took a sabbatical research leave from the University of Calgary and pursued the technology with Wachsman and his team at the University of Maryland.
“Professor Thangadurai’s sabbatical at UMD was a turning point in all of our research,” says Wachsman, director of UMD’s Energy Research Center. “That collaboration enabled us to invent the needed breakthrough in solid-state batteries that not only overcomes the inherent flammability issues with today’s batteries, but is resulting in the development of a truly revolutionary smaller, lighter, and lower-cost battery to meet the rapidly growing need for energy storage.”
The team’s next steps are to scale up the size of their battery and study the long-term stability of the materials.
Thangadurai expects it will take about five years to develop a commercial-scale, solid-state lithium battery incorporating their technology.
The team’s research was supported by a contract with the U.S. Department of Energy.
“This is an example of an international collaboration opportunity, where the University of Calgary and the University of Maryland, through the U.S. Department of Energy, are developing energy storage technologies for the next generations,” Thangadurai says.